心外科手术由于其复杂性、高风险性等特点,使围术期凝血功能管理、输血管理和抗凝药物疗效监测等临床活动面临巨大挑战。血栓弹力图作为一种新型的基于检测血液粘弹性特性的凝血功能监测系统,在自其产生以来的数十年间,在围术期管理与输血管理方面已经显示出其优越于传统凝血功能检测的特性。血栓弹力图系统的各项组合均可能应用于心外科的相关手术中。
血栓弹力图仪(TEG)可实时检测手术期间病人的凝血功能状态,协助外科医生和麻醉师对病人输血治疗的时间、输注血液制品的种类以及输注量等随时做出适当的决定,尤其对那些高出血风险或凝血障碍的手术病人,具有更重要的应用价值。在过去的几十年里,血栓弹力图仪的应用领域主要集中在围术期病人的输血指导和管理[1-4]。
一、血栓弹力图概述
于 1948 年由德国人 Hartert 发明。其原理是在模拟人体内环境下凝血-纤溶整个过程的同时,通过物理方法将血块弹性强度转换成图形表示,通过输出一系列参数从而能直观判断凝血情况并分析成因。
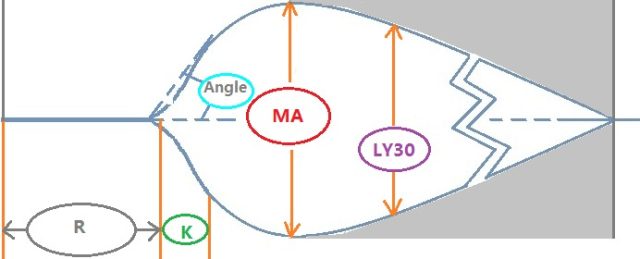
图 1. 血栓弹力图检测结果示意图
二、血栓弹力图在心外科的应用
(1)输血管理
出血是心脏外科手术的严重并发症之一,而导致患者出血的原因多种多样,包括手术的复杂性、术前抗血小板和/或抗凝治疗、术后肝素的持续效果、凝血因子和血小板的消耗与稀释等[5]。此外,心脏外科手术中使用体外循环、抗凝剂和血液制品等也会导致或加重凝血功能障碍,对患者的预后产生不利的影响[6-8]。Besser 等报道,英国的心胸外科手术大约消耗英国国家血液中心 10% 的血液制品,其中 90% 应用于大约仅 10% 的患者[8]。血栓弹力图是一种新型的适用于床旁检测的血液粘弹性检测系统(Viscoelastic Coagulation Assay, VCA),使用全血标本,监测血液凝固和纤溶的全过程,比传统凝血功能检测更符合现代以细胞学为基础的凝血反应机制。从上世纪 50 年代后期血栓弹力图被用于心脏外科手术以来,大量的临床数据证明血栓弹力图已经成为心脏外科手术中一项必备的辅助检测手段,尤其是在监控心外科手术患者围术期凝血功能、指导血液制品的输注方面具有重要的临床意义[9-20]。一般来讲,患者的血样要以鱼精蛋白中和肝素,并调整到最佳温度、pH 值和离子强度后,再进行血栓弹力图检测;不过在高出血风险手术时(如再次手术、循环辅助、心脏移植等),可以更系统地使用血栓弹力图检测。
(2)围术期凝血功能管理
血栓弹力图应用于围术期凝血功能管理的历史由来已久。早在 1985 年,血栓弹力图已在肝移植术中进行凝血功能监测[42],且历经 30 多年发展,血栓弹力图在围术期的应用越来越普遍[43-45]。心外科手术由于其风险大、难度高,其围术期的凝血功能情况更为复杂和危险。据统计,在 40 岁以上人群非择期手术的案例中,发生术后心梗(Preioperative Myocardial Infarction, PMI)或死亡的概率高达 2.5%,而在心血管手术中这个比例高达 6.2%[46]。EACTS(European Journal of Cardio-Thoracic Surgery)指南中明确指出心外科手术的围术期管理内容包括[47]:
确定患者是否具有术后出血的风险;
围术期输血管理及贫血的治疗;
术后血栓风险的评估及抗血栓治疗的疗效监测。
早在 1987 年,已有血栓弹力图应用于心外科手术的报道[48]。2012 年时学者 McCrath 的综述汇总了当时已经发表的 16 个研究报告,结果表明血栓弹力图比传统凝血功能检在预测手术出血、减少二次手术机率方面更加具有优越性[49]。正因如此,2014 年时 NICE(The National Institute for Health and Care Excellence)推荐使用血栓弹力图检测进行心外科手术的围术期管理[50]。
(3)抗血小板药物疗效监测
无论是急性的还是择期的心外科手术,术后的抗血小板药物治疗几乎必不可少(表 4)。服用抗血小板药物的目的是预防术后血栓形成,但抗血小板药物的风险在于出血,因此临床上对于正在服用抗血小板药物的患者必须进行紧密的凝血功能监测。
表 4. 抗血小板药物在心外科的推荐级别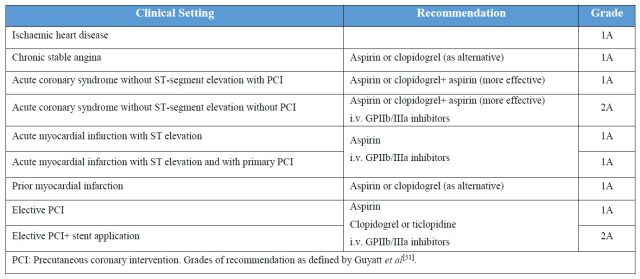
以往临床上常用血小板功能分析仪/聚集分析仪(PFA-100,VerifyNow 等)监测血小板对药物的反应,但检测费时、重复性差等结果导致临床希望更有效的手段检测抗血小板药物疗效。血栓弹力图里的血小板图试剂盒为临床提供了新的基于血小板反应性的个性化检测方法[52, 53]。此外,参数 MA 还可用于基于治疗窗的个体化抗血小板治疗,在氯吡格雷使用过程中,MA(ADP)范围维持在 31~47 中被认为是一个合理的安全范围,可以有效平衡药效和合理的安全[54]。血小板图实验中参数 MA 还能帮助评估总体的血小板功能,预测再缺血事件的发生风险[55],还能帮助预估停药时间[56],而对入院时已在服用氯吡格雷的非紧急手术的患者,还可以帮助预测最佳的手术时间[57]。血小板图试剂盒有三组合:AA、ADP、AA+ADP。用以监测单独或联合使用 AA 途径(阿司匹林)或 ADP 途径(氯吡格雷)的抗小板药物疗效。结果以血小板抑制率表示(表 5):
表 5. 血小板图试验结果解读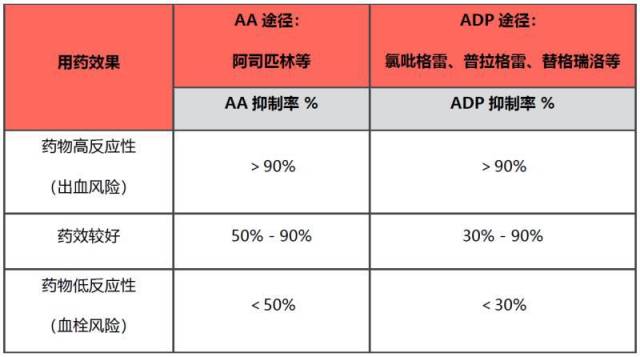
三、血栓弹力图的局限性
据报道[7],在 CPB(Cardio Pulmonary Bypass)时,患者体温常低于 35℃,平均 32℃,此温度下凝血因子和血小板的活性大大受到影响。但血栓弹力图的检测是将血液标本复温到 37℃ 再进行检测,此时已经不能反映患者真实的凝血功能情况。且由于检测原理所限,血栓弹力图也不能反映血管内皮的功能情况。这些原因均可能影响了血栓弹力图在心外科中的应用。不过,临床活动中情况复杂,凝血功能检测方法种类虽多,但各有不足之处,现在很难讲哪个方法是最好,而是需要在临床活动中将不同的检测方法和结果参数进行有序有理的组合,才能帮助临床医生应对「难缠」的凝血异常问题。
TIPS:
四、参考文献
1. Shen, L., S. Tabaie, and N. Ivascu, Viscoelastic testing inside and beyond the operating room. J Thorac Dis, 2017. 9(Suppl 4): p. S299-S308.
2. Wikkelso, A., et al., Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia, 2017. 72(4): p. 519-531.
3. GJ., S.G.M., Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. Br J Anaesth., 2017. 118(6): p. 823-33.
4. Fahrendorff, M., R.S. Oliveri, and P.I. Johansson, The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - A systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med, 2017. 25(1): p. 39.
5. Murphy GJ, e.a., Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. , 2007. 116: p. 2544-52.
6. Klein., B.M.A., The coagulopathy of cardiopulmonary bypass. Critical Reviews in Clinical Laboratory Sciences., 2010. 47(5-6): p. 197-212.
7. Ranucci M, e.a., Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Annals of Thoracic Surgery., 2013. 96(2): p. 478-85.
8. Besser MW, e.a., Haemostatic management of cardiac surgical haemorrhage. Anaesthesia. , 2015. 70(S1.): p. 81-e31.
9. Conte AH, e.a., Thromboelastrography (TEG) is still relevant in the 21st century as a point-of-care test for monitoring coagulation status in the cardiac surgical suite. . Seminars in Cardiothoracic and Vascular Anesthesia., 2017. 21(3): p. 212-6.
10. Bhardwaj V, e.a., Coagulopathies in cyanotic cardiac patients: An analysis with three point-of-care testing devices (Thromboelastography, rotational thromboelastometry, and sonoclot analyzer). Ann Card Anaesth., 2017. 20(2): p. 212-8.
11. Ranucci M, e.a., The effectiveness of 10 years of interventions to control postoperative bleeding in adult cardiac surgery. Interact Cardiovasc Thorac Surg., 2017. 24(2): p. 196-202.
12. Fabbro M, e.a., Comparison of Thrombelastography-Derived Fibrinogen Values at Rewarming and Following Cardiopulmonary Bypass in Cardiac Surgery Patients. Anesth Analg. , 2016. 123(3): p. 570-7.
13. Rafiq S, e.a., Preoperative hemostatic testing and the risk of postoperative bleeding in coronary artery bypass surgery patients. J Card Surg., 2016. 31(9): p. 565-71.
14. Yildirim F, e.a., Thromboelastogram reduces blood use by inspecting coagulation in heart surgery. Asian Cardiovasc Thorac Ann., 2016. 24(5): p. 441-4.
15. YL Iyer , P.H., L Mcnicol , L Weinberg., The effects on coagulation of the reinfusion of unprocessed residual blood from the cardiopulmonary bypass. BMC Res Notes., 2016. 9(1): p. 61.
16. M Pedersen, M.K., AM Hvas , HB Ravn., Autotransfusion of a restricted volume of shed mediastinal blood does not affect the haemostatic capacity in patients following cardiac surgery. Scand J Clin Lab Invest. , 2015. 75(4): p. 314-8.
17. Hans GA, e.a., Impact of 6 % hydroxyethyl starch (HES) 130/0.4 on the correlation between standard laboratory tests and thromboelastography (TEG®) after cardiopulmonary bypass. Thromb Res., 2015. 135(5): p. 984-9.
18. Mishra PK, e.a., The role of point-of-care assessment of platelet function in predicting postoperative bleeding and transfusion requirements after coronary artery bypass grafting. . Ann Card Anaesth., 2015. 18(1): p. 45-51.
19. Riley JB, e.a., Coagulation Parameter Thresholds Associated with Non-Bleeding in the Eighth Hour of Adult Cardiac Surgical Post-Cardiotomy Extracorporeal Membrane Oxygenation. . J Extra Corpor Technol. , 2016. 48(2): p. 71-8.
20. Nair P, e.a., Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. . J Cardiothorac Vasc Anesth. , 2015. 29(2): p. 288-96.
21. Analg., A., Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg., 1999. 88(2): p. 312-9.
22. Agnese Ozolina, E.S., Indulis Vanags., The Predictive Value of Thrombelastography and Routine Coagulation Tests for Postoperative Blood Loss in Open Heart Surgery. ACTA HIRURGICA LATVIENSIS, 2010. 10(2): p. 5.
23. Ak, K., et al., Thromboelastography-based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg., 2009. 24(4): p. 404-10.
24. Deppe AC, e.a., Point-of-care thromboelastography/thromboelastometry based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. . J Surg Res. , 2016. 203(2): p. 424-33.
25. Karkouti K, e.a., Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. , 2016. 134: p. 1152-62.
26. Miller BE, e.a., Functional maturity of the coagulation system in children: an evaluation using thrombelastography. Anesth Analg. , 1997. 84: p. 745-8.
27. Chan K-L, e.a., Reference values for kaolin-activated thromboelastography in healthy children. . Anesth Analg. , 2007. 105: p. 1610-3.
28. Edwards RM, e.a., Parameters of thromboelastography in healthy newborns. Am J Clin Pathol. , 2008. 130: p. 99-102.
29. Sewell EK, e.a., Thromboelastography in term neonates: an alternative approach to evaluating coagulopathy. Arch Dis Child Fetal Neonatal Ed. , 2017. 102: p. F79-84.
30. Sokou R, e.a., Reference ranges of thromboelastometry in healthy full-term and pre-term neonates. . Clin Chem Lab Med., 2017. 55: p. 1592-7.
31. Haizinger B, e.a., Activated thrombelastogram in neonates and infants with complex congenital heart disease in comparison with healthy children. Br J Anaesth., 2006. 97: p. 545-52.
32. Kim JY, e.a., Reference intervals of thromboelastometric evaluation of coagulation in pediatric patients with congenital heart diseases: a retrospective investigation. Med Sci Monit Int Med J Exp Clin Res . 2016. 22: p. 3576-87.
33. Romlin BS, e.a., Intraoperative thromboelastometry is associated with reduced transfusion prevalence in pediatric cardiac surgery. Anesth Analg., 2011. 112: p. 30-6.
34. Faraoni D, e.a., Evaluation of dynamic parameters of thrombus formation measured on whole blood using rotational thromboelastometry in children undergoing cardiac surgery: a descriptive study. Paediatr Anaesth. , 2015. 25(6): p. 573-9.
35. Nakayama Y, e.a., Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth. , 2015. 114(1): p. 91-102.
36. Northrop MS, e.a., The use of an extracorporeal membrane oxygenation anticoagulation laboratory protocol is associated with decreased blood product use, decreased hemorrhagic complications, and increased circuit life. . Pediatr Crit Care Med. , 2015. 16(1): p. 66-74.
37. Meier PM, e.a., Multivariable predictors of substantial blood loss in children undergoing craniosynostosis repair: implications for risk stratification. Paediatr Anaesth., 2016. 26(10): p. 960-9.
38. Vida VL, e.a., The Coagulative Profile of Cyanotic Children Undergoing Cardiac Surgery: The Role of Whole Blood Preoperative Thromboelastometry on Postoperative Transfusion Requirement. . Artif Organs., 2016. 40(7): p. 698-705.
39. Kane LC, e.a., Thromboelastography--does it impact blood component transfusion in pediatric heart surgery? J Surg Res., 2016. 200(1): p. 21-7.
40. Rizza A, e.a., Kaolin-activated thromboelastography and standard coagulation assays in cyanotic and acyanotic infants undergoing complex cardiac surgery: a prospective cohort study. . Paediatr Anaesth., 2017. 27(2): p. 170-80.
41. Gautam NK, e.a., Performance of functional fibrinogen thromboelastography in children undergoing congenital heart surgery. Paediatr Anaesth. , 2017. 27(2): p. 181-9.
42. Kang, Y.G., et al., Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg., 1985. 64(9): p. 888-96.
43. Alexander Hincker, J.F., Robert N Sladen and Gebhard Wagener., Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Critical care (London, England). 2014. 18(5): p. 549.
44. Kasivisvanathan., R., A clinician's guide to viscoelastic testing in the perioperative period. British journal of hospital medicine (London, England: 2005). 2015. 76(12): p. 690-5.
45. Toukh M, S.D., Black A, Robb S, Leveridge M, Graham CH et al. , Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thromb Res . 2014. 133: p. 88-95.
46. Don Poldermans, M., et al., Pre-Operative Risk Assessment and Risk Reduction Before Surgery. Journal of the American College of Cardiology., 2008. 51(20): p. 1913-24.
47. Sousa-Uva, M., et al., The 2017 EACTS guidelines on perioperative medication in adult cardiac surgery and patient blood management. Eur J Cardiothorac Surg, 2018. 53(1): p. 1-2.
48. Spiess BD, T.K., Thromboelastography as an indicator of post-cardiopulmonary bypass coagulopathies. J Clin Monit., 1987. 3(1): p. 25-30.
49. McCrath DJ, C.E., Frumento RJ, Hirsh AL, Bennett-Guerrero E., Coagulopathy and hemostatic monitoring in cardiac surgery: An update. Scandinavian cardiovascular journal: SCJ., 2012. 46(4): p. 194-202.
50. NICE., Detecting, Monitoring and Managing Haemostasis: Viscoelastometric Point-of Care Testing (ROTEM, TEG and Sonoclot Systems). NICE Diagnostics Guidance 13. . 2014 National Institute for Health and Care Excellence, London. , 2014.
51. Guyatt G, e.a., Grade of recommendation for antithrombotic agents. Chest., 2001. 119: p. 3S-7S.
52. Swallow, R.A., et al., Thromboelastography: potential bedside tool to assess the effects of antiplatelet therapy? Platelets., 2006. 17(6): p. 385-92.
53. Sambu., N., Personalised antiplatelet therapy in stent thrombosis: Observations from the Clopidogrel Resistance in Stent Thrombosis (CREST) registry. Heart (British Cardiac Society), 2012. 98(9): p. 706-11.
54. Tantry, U.S., et al., Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol, 2013. 62(24): p. 2261-73.
55. Gurbel, P.A., et al., Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol, 2005. 46(10): p. 1820-6.
56. Mahla, E., et al., Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ Cardiovasc Interv, 2012. 5(2): p. 261-9.
57. Cattano, D., et al., Perioperative assessment of platelet function by Thromboelastograph Platelet Mapping in cardiovascular patients undergoing non-cardiac surgery. J Thromb Thrombolysis, 2013. 35(1): p. 23-30.
五、联系我们
广州阳普医疗科技股份有限公司
地址:广东省广州市经济技术开发区科学城开源大道 102 号
电话:020-32312518
服务专线:4009300030
邮箱:mkd@improve-medical.com
官网:www.improve-medical.com
